Is Antibody Test For Covid 19 Fda Approved
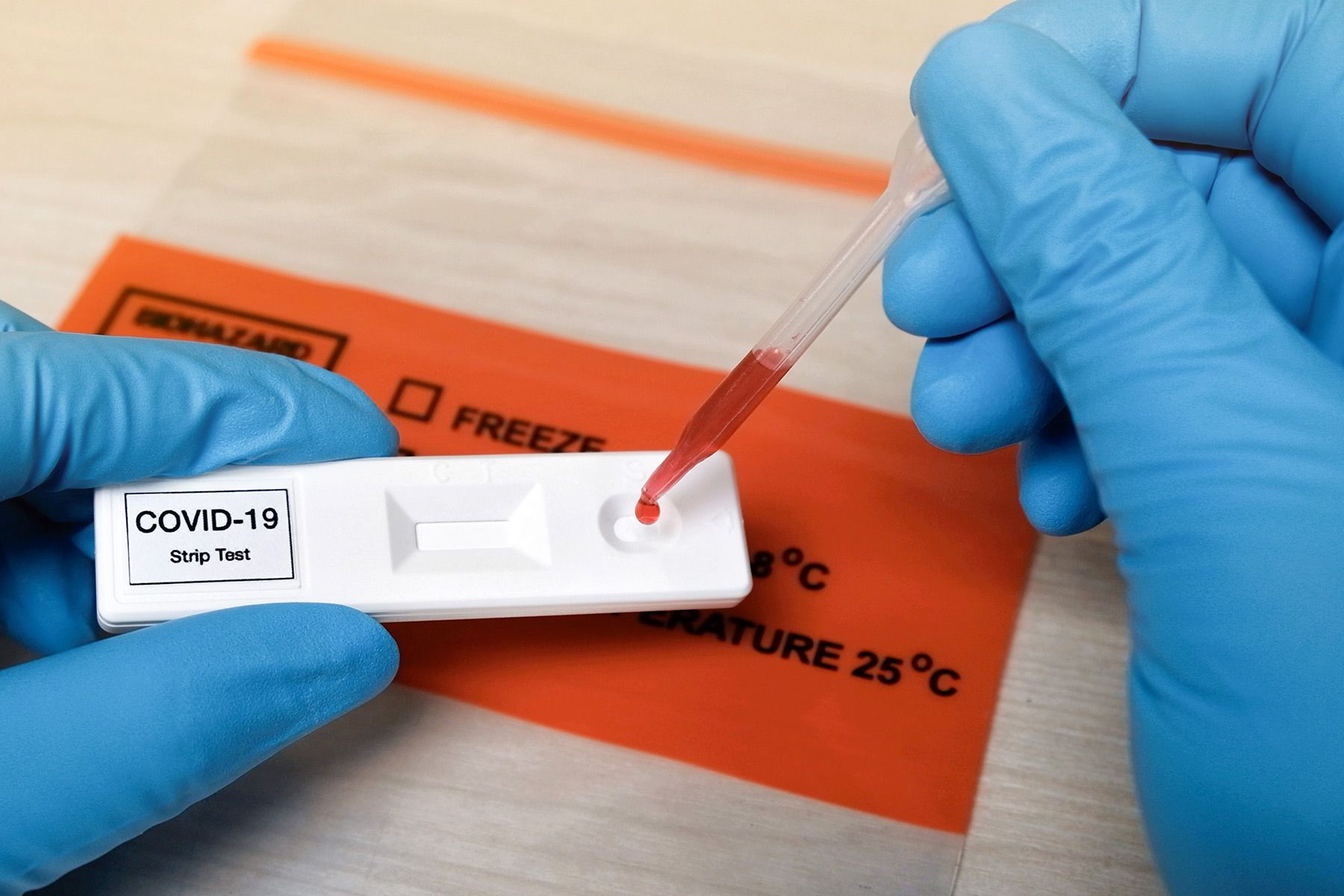
Antibody tests are used to detect antibodies to the COVID-19 virus to see if its likely that you have had the virus before. The Food and Drug Administration FDA approves the use of five 5 Rapid Test Kits for COVID-19 today March 30 2020.
A Reality Check On Antibody Testing How Do We Race Forward Thoughtfully Abc News
519 صفوف Coronavirus COVID-19 Update.

Is antibody test for covid 19 fda approved. This is different than assays that test for presence of the virusthose test to determine if a patient has COVID-19. Food and Drug Administration FDA approved the first blood test that looks for the antibodies against the novel coronavirus that causes COVID-19. The test works by taking a blood sample and testing for the presence of.
FDA Advisory No. You can get the antibody test through your doctor or healthcare provider. FDA Approves 1st COVID-19 Antibody Test.
First Antibody Test for COVID-19 Gets FDA Authorization Emergency use OKd to diagnose infection by Molly Walker Associate Editor MedPage Today. Since then the regulatory agency has allowed more than 100 antibody tests onto the market without full review and that has proven to become a problem as some tests are not as accurate as hoped. The Food and Drug Administration FDA has granted an emergency use authorization EUA for the first point-of-care antibody test for COVID-19.
Food and Drug Administration FDA has approved for emergency use. This COVID-19 semi-quantitative test is for individuals who think they may have had COVID-19 and do not currently have symptoms. These point-of-care test kits are registered for use in countries with reliable regulatory agencies such as China and Singapore.
The test checks for protective antibodies in a. The Food and Drug Administration gave emergency approval to a COVID-19 antibody test that boasts near-perfect accuracy the company said Sunday. But what about the tests the US.
We approve kits that are registered and. 2020-483 FDA APPROVES RAPID ANTIBODY TEST KITS FOR COVID-19. In line with the ongoing response to the increasing number of COVID-19 cases in the Philippines the Food and Drug Administration FDA- Philippines hereby provides an initial list of approved Rapid Antibody Test Kits.
The FDA website lists authorized tests and tests offered under FDA COVID-19 diagnostic policy guidance. Doctors say COVID-19 antibody tests arent FDA approved should be taken with caution Thousands of people are paying out of pocket to take an antibody test at private labs in the valley. This limits a tests effectiveness for diagnosing COVID-19 and is why it should not be used as the sole basis to diagnose the disease.
The FDA supports all efforts to address this pandemic. How useful or accurate are those tests. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19 the first antibody test released amidst the pandemic.
The FDAs Experience with Covid-19 Antibody Tests Early in the pandemic the FDA recognized that ensuring access to antibody tests could advance understanding of. The FDA approved PerkinElmers antibody test for emergency use just days after issuing another significant EUA for remdesivir an antiviral medication produced by. DETROIT The US.
FDA Authorizes First Point-of-Care Antibody. In people who have received a COVID-19 vaccination antibody testing is not recommended to determine whether you are immune or protected from COVID-19. Food and Drug Administration is tightening the rules on coronavirus COVID-19 antibody testing.
In a recent post we warned readers against rushing out to purchase one of the many COVID-19 antibody tests suddenly flooding the market noting that few of these tests have been independently validated and many are grossly inaccurate. The Assure COVID-19 IgGIgM Rapid Test Device a simple fingerstick blood test was previously used for laboratory testing to detect antibodies to SARS-CoV-2 the virus that causes COVID-19 in patients. Antibodies may not be present in detectable levels in early days of an infection.
Officials have issued a new. Food and Drug Administration FDA approved the first antibody test for COVID-19. HealthDayThe first COVID-19 virus antibody test for use in the United States has been approved by the Food and Drug Administration.
At the beginning of April the US.
Coronavirus Covid 19 Antibody Tests How It Works How To Get One
Rapid Covid 19 Antibody Test Fda Eua Authorized Made In Usa Amazon Com Industrial Scientific
Fda Grapples With Uneven Mix Of Coronavirus Antibody Tests Los Angeles Times
Arkansas Company S Covid 19 Antibody Test Approved By Fda Katv
Us Fda Releases List Of Withdrawn Covid 19 Antibody Tests
Roche Snags Fda Nod For Covid 19 Antibody Test 2020 05 04 Bioworld
Fears Of Wild West As Covid 19 Blood Tests Hit The Market Abc News
Covid 19 Antibody Testing Locations In Las Vegas E7 Health E7 Health
Covid 19 Tests And Collection Kits Authorized By The Fda In 2020 Infographic Fda



Posting Komentar untuk "Is Antibody Test For Covid 19 Fda Approved"